


In addition to our clinical stage program in LipoCurc™ for the treatment of cancer, we are developing our CorreQT technology for treatment of cardiac arrhythmia, and the mitigation of cardiomyopathy associated with chemotherapy.
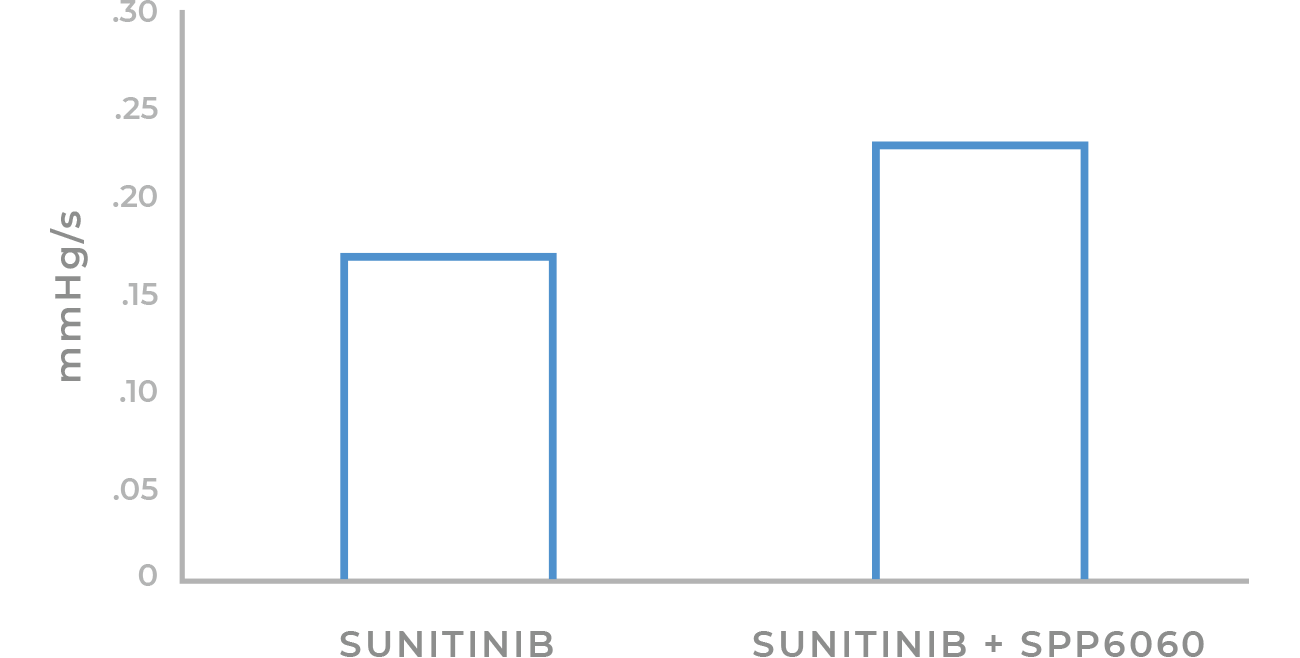
LV Rate of Contraction
In animal studies, guinea pigs who were administered chemotherapy showed significantly greater heart function when the chemotherapy was co-administered with Signpath’s CorreQT adjuvant.
CorreQT technology protects the heart from damage caused by chemotherapy
Protecting the Heart from Damage During Chemotherapy
Using the CorreQT technology platform, Signpath is developing a drug which protects the heart from damage caused by administration of chemotherapy agents. Pre-clinical data shows that Signpath compounds are effective in mitigating the heart muscle damage which is a common side effect of cancer chemotherapy. In addition, testing confirms that Signpath’s compound does not interfere with the anti-cancer efficacy of the chemotherapy agent. Signpath is working on compound optimization for this cancer supportive care indication. The goal is to create a cardio-protective agent that is widely used in conjunction with a broad spectrum of chemotherapy agents (a cancer adjuvant model similar to Amgen’s Neupogen.) The cancer supportive care drug market was $22.9 billion in 2015.

