Curcumin-like compounds are found in a genus of root vegetables that includes turmeric and ginger; they have been consumed for thousands of years and epidemiological evidence suggests various health benefits. Historically, development of Curcumin as a pharmaceutical product has been hampered by Curcumin’s two major flaws; poor absorption and cardiac side effects. Curcumin in its natural form is very poorly absorbed and only very limited amounts get into the body when administered orally. If Curcumin is administered intravenously to achieve higher blood levels, it causes significant cardiac arrhythmia side effects. Signpath’s proprietary liposomal synthetic Curcumin formulation (LipoCurc™) solves both of these issues, achieving high bioavailability (over 1000 times higher than oral Curcumin) without causing cardiac arrhythmia side effects.


2
70
II
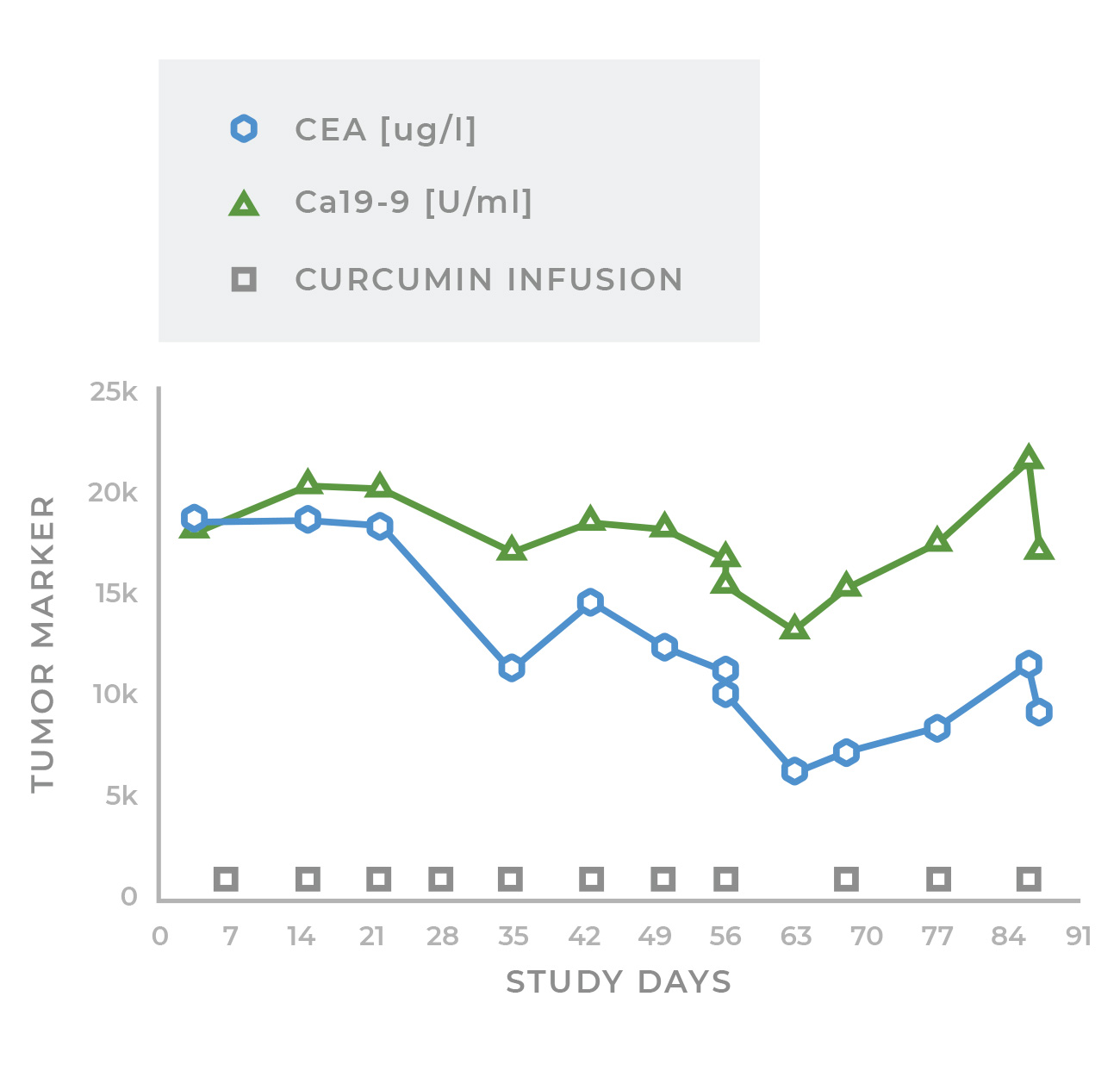
A patient with metastatic colon cancer who had received seven prior lines of multi-drug chemotherapy exhibits significant reduction in cancer markers as a result of LipoCurc treatment
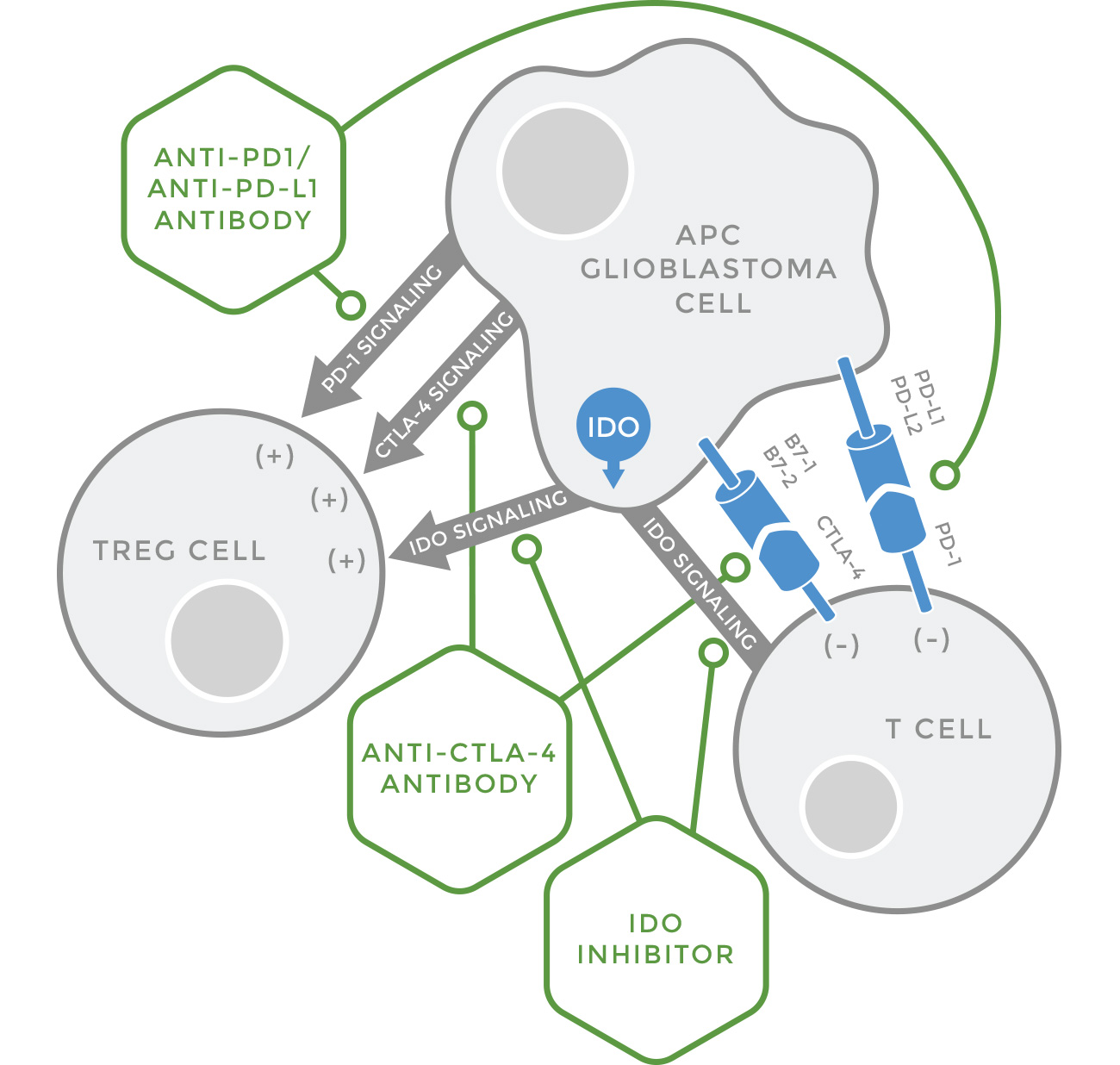
Mechanisms of Glioblastoma-Induced Immunosuppression
Glioblastomas and other cancers adapt to treatment (chemotherapy, radiation therapy, vaccines, CAR-T therapy) by inducing severe immunosuppression in the patient, and are thus able to escape the effects of treatment. They secrete proteins (PD-L1, PD-L2, B7), which can bind to T cells and signal a reduced immune response, or enzymes (IDO) which cause unbalanced tryptophan metabolism and production of immunotoxic metabolites. Immune checkpoint inhibitors on the market block either the binding of PD-L1 or PD-L2 to PD-1 on the T cell or the binding of B7 to CTLA-4 , Liposomal curcumin blocks each of these immunosuppessive pathways and also reduces the production of IDO.
Cancer Indications Currently In Clinical Development
Signpath has successfully completed Phase 1a and Phase 1b human clinical trials using LipoCurc™. These clinical trials have shown LipoCurc™ to be safe at clinical doses, with minimal adverse side effects. The Phase 1b trial showed significant signs of efficacy in several heavily pre-treated salvage patients.
Two very heavily pretreated patients had major reductions in cancer markers. A patient with metastatic colon cancer who had received seven prior lines of multi-drug chemotherapy had a reduction in his CEA from 18,542 to 6,441 and a decrease in CA 19-9 from 18,105 to 13,238. A patient with metastatic prostate carcinoma who had also received seven prior lines of therapy showed a reduction of his PSA from 649 to 350 and of his LDH from 435 to 220. It is very rare to see evidence of response in cancer patients with solid tumors even after four or five previous lines of therapy.
Using the data generated from the Phase 1 trials, we have initiated a Phase 2 trial in glioblastoma.
Glioblastoma as a Target for Liposomal Curcumin
Our Phase 2 trial in first line glioblastoma patients is currently underway.
Glioblastoma, is the most common primary brain tumor, and has few available therapies providing significant improvement in survival. Glioblastoma has the highest number of cases of primary malignant tumors of the brain, with over 14,000 new cases annually in the United States. Additionally, glioblastoma patients have limited survival with current treatment, allowing for rapid evaluation of endpoint criteria. The market for glioblastoma alone is projected at over $3 billion by 2024.
Unlike most pharmaceutical agents, LipoCurc™ passes the blood/brain barrier and is able to access brain tissue. LipoCurc™ acts through multiple pathways, including immunomodulation. Glioblastoma tumors are not normally sensitive to the body’s natural immunity. Glioblastomas adapt to treatment by causing secretion of immune checkpoint molecules that use four major pathways (PD-L1/PD-1; PD-L2/PD-1; B7/CTLA4; IDO) to prevent the patient from mounting an immune response. Unlike other immuno-oncology agents, LipoCurc™ has minimal side effects.
Mesothelioma as a Target for Liposomal Curcumin
Mesothelioma is a cancer that arises from the mesothelium, the thin lining that surrounds the lungs (pleural mesothelioma) or the abdomen (peritoneal mesothelioma). In rare instances, this cancer can develop in the sacs surrounding the heart of the testis. Most cases are caused by exposure to asbestos.
Curcumin has shown anti-mesothelioma activity in multiple experimental models. However, oral curcumin is very poorly absorbed into the body. With intravenous LipoCurc, we can achieve curcumin blood levels over 1000 times that achieved with standard oral curcumin. The liposomal component not only allows us to achieve these levels, it protects against cardiac, pulmonary, renal and hepatic toxicity and greatly contributes to the efficacy.
We are preparing for a Phase 2 trial where we will inject LipoCurc through the chest wall directly into the pleural cavity, which is the potential space between the lung and the chest wall where mesothelioma develops. It is also the area where other cancers, particularly breast cancers, frequently metastasize. We have shown that treatment with LipoCurc directly through the chest wall is safe, and will allow us to deliver liposomal curcumin directly to the site of the cancer.
Mechanism of Action
The mechanism of action of LipoCurcTM is in part due to curcumin activation of sphingomyelinase leading to an increased ceramide/S1P ratio. The increased ceramide activates growth inhibition and cell death mechanisms. Ceramide is an N-acylsphingosine consisting of a fatty acid bound to the amino group of the sphingoid base, sphingosine. As an agent of cell signaling, ceramide activates a variety of protein kinases. Differential activation of these proteins determines the nature of the signal and its effect on the cell. Generally, in cancer cells ceramides favor anti-proliferative and cell death pathways (such as senescence and apoptosis). Curcumin stimulates ceramide production, which leads to cancer cell death through apoptosis.
Curcumin also has an inhibitory effect on tumor stem cells which may contribute to its proposed clinical benefit.
There are molecular based reasons for using liposomal curcumin as a treatment for cancer. Brain tumors, including glioblastoma, exhibit distinct patterns of cancer gene mutations of the IDH1 gene, which functions as a tumor suppressor. IDH1 gene mutations have been shown to drive tumor formation and progression in glioblastomas. When the IDH1 gene is mutated, it causes over expression of Hypoxia-inducible factor 1 (HIF-1) which stimulates tumor growth and metastasis. Curcumin inhibits over expression of HIF-1.
Osteopontin (OPN) is a secreted protein involved in most aspects of tumor progression and metastasis development. Elevated OPN expression has been reported in the highest grade and most aggressive glioblastomas. Curcumin inhibits osteopontin, which provides another rationale for anti-tumor effect.

