Signpath has developed a unique, patented platform technology that makes existing drugs safer by eliminating their cardiac side effects. This platform is referred to as the CorreQT technology. The CorreQT technology allows for creation of proprietary lipid based drugs which mitigate cardiac side effects when co-administered with drugs that cause cardiotoxicity.
One of Signpath’s CorreQT compounds, SPP4040, has been shown to mitigate the cardiomyopathy (heart muscle damage) which is a common side effect of cancer chemotherapy. Signpath is developing SPP4040 as a cancer supportive care drug, for protection of the heart during chemotherapy.
Another application of the CorreQT technology is the prevention of cardiac arrhythmia, which is a common and dangerous side effect of many drugs.
In vivo testing in rats, guinea pigs, and rabbits, has shown that administration of a CorreQT adjuvant concomitantly with a target drug which causes cardiac arrhythmia, will eliminate the dangerous arrhythmia side effect induced by the target drug. This mitigation of cardiac arrhythmia has been observed in conjunction with various classes of compounds, including anti-cancer agents, antibiotics, antipsychotics, antihistamines and other drugs. To date, the CorreQT adjuvants have been successful in eliminating QT prolongation when given in combination with every target drug tested (~50 different drugs.)

290+
10+
$5+
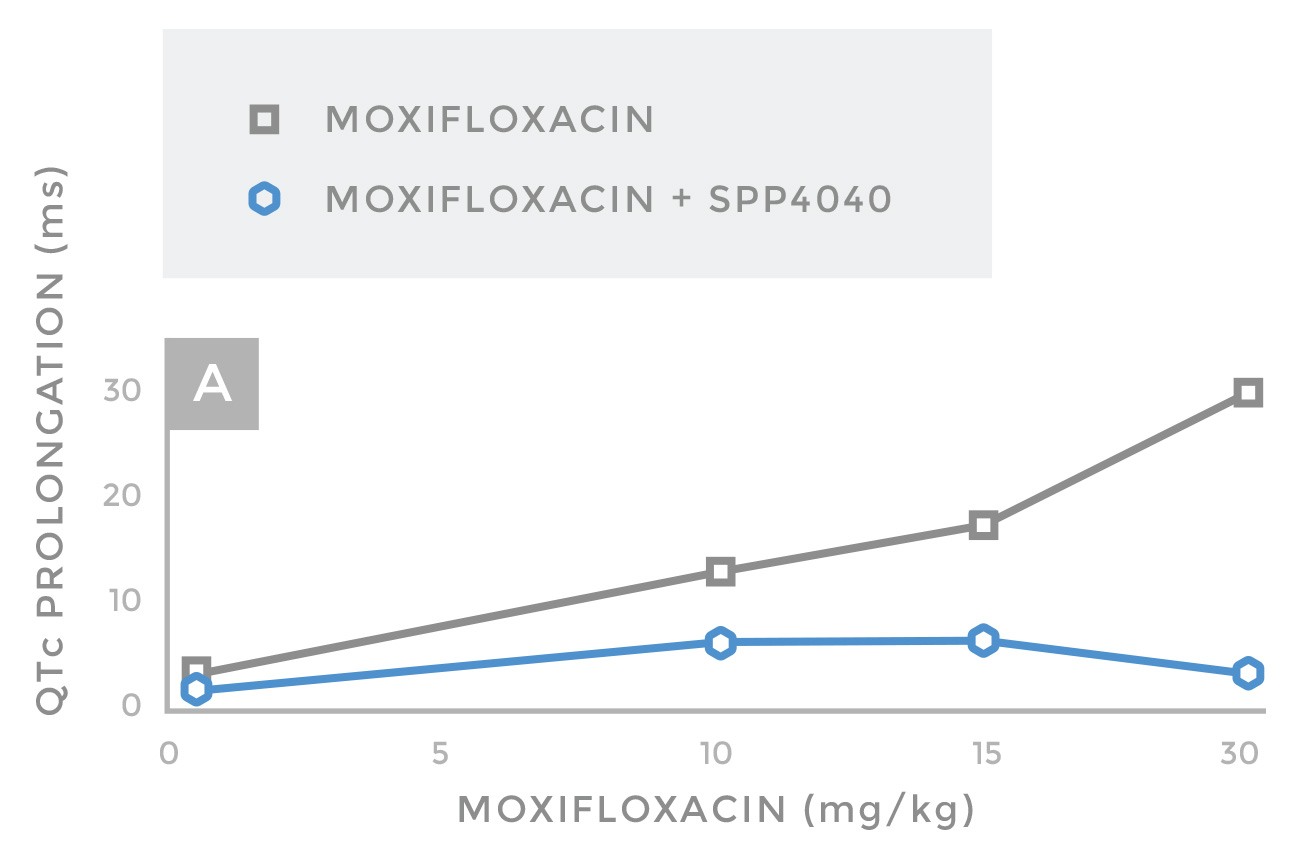
Chart A
Increasing oral doses of Moxifloxacin induced dose-dependent QTc prolongation in guinea pigs, as they do in human patients. (Moxifloxacin is the standard for QTc prolongation assessment in clinical trials, due to its highly predictable dose-response curve.) Since a 30-ms QTc prolongation increases the risk of Torsades de Pointes significantly in these animals, it was selected as the threshold for pro-arrhythmic concern. In identical experiments, Moxifloxacin was administered concomitantly with oral SPP4040 at a 1:1 ratio. The guinea pigs exhibited negligible (almost 0 ms) QT prolongation when dosed with the two compounds.
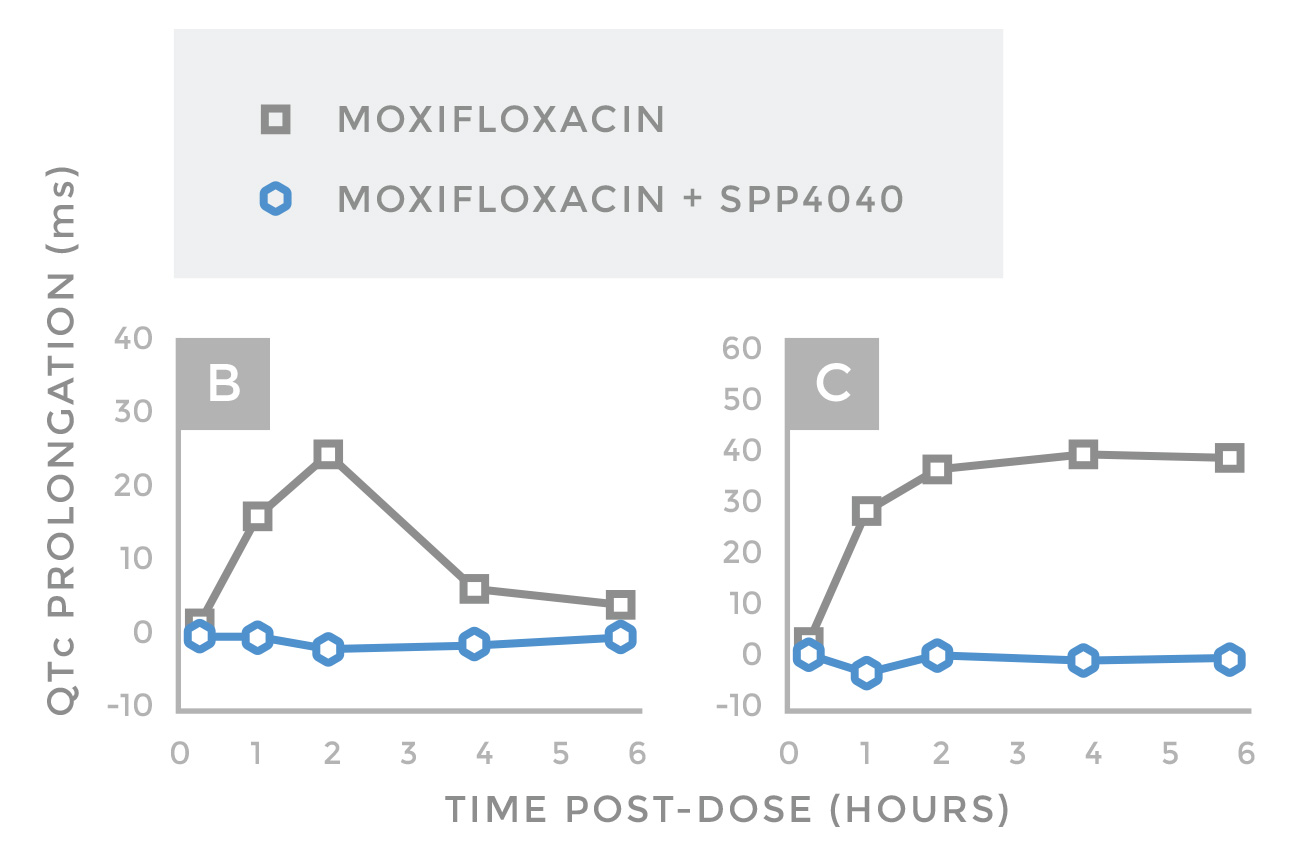
Chart B & C
Monitoring of QTc prolongation by 20 mg/kg oral Moxifloxacin with, and without, 20 mg/kg oral SPP4040. Moxifloxacin caused a maximal QTc prolongation of 25-30 ms approximately 2-hours post-dose; The effect was transient in guinea pig, but sustained in rabbits. In contrast, Moxifloxacin failed to prolong the QTc over the entire 6 hours of monitoring in animals having been dosed with SPP4040, confirming the successful mitigation of QTc prolongation by Moxifloxacin, a drug renowned for its predictable QTc prolonging abilities. Importantly because SPP4040 requires some enteric metabolism, the effect was also observed in rabbits, which are characterized by a more human-like gastro intestinal function than guinea pigs.
Development Strategy; Arrhythmia
Mitigation of Cardiac Arrythmia
Signpath can improve drugs with proven efficacy but which are limited by cardiac arrhythmia side effects. Clinical testing is focused on a single, objectively measurable safety side effect (QT prolongation.) There is no significant competition in this arena. No other companies have drugs which treat broad-spectrum drug-induced cardiac arrhythmia. Cardiac arrhythmia animal models are highly correlated with human results (as high as 90%.)
Development Strategy; Cardio-Oncology
Currently there are no drugs available which effectively mitigate the cardio-toxic effects of cancer treatment.
SignPath is developing a first-in-class cardio-oncology drug which will be administered to cancer patients; protecting their heart from the harm caused by anti-cancer drugs.
This unique cardio-oncology therapy has the potential to be used broadly across many different cancer indications in conjunction with many different cancer drugs.

